
Inspired by the electric shock capabilities of electric eels, scientists have developed a soft, stretchable "jelly" battery ideal for wearable devices or soft robotics, according to a new paper published in the journal Science Advances. With further testing in living organisms, the batteries might even be useful as brain implants for targeted drug delivery to treat epilepsy, among other conditions.
As previously reported, the electric eel produces its signature electric discharges—both low and high voltages, depending on the purpose for discharging—via three pairs of abdominal organs composed of modified muscle cells called electrocytes, located symmetrically along both sides of the eel. The brain sends a signal to the electrocytes, opening ion channels and briefly reversing the polarity. The difference in electric potential then generates a current, much like a battery with stacked plates.
Vanderbilt University biologist and neuroscientist Kenneth Catania is one of the most prominent scientists studying electric eels these days. He has found that the creatures can vary the degree of voltage in their electrical discharges, using lower voltages for hunting purposes and higher voltages to stun and kill prey. Those higher voltages are also useful for tracking potential prey, akin to how bats use echolocation. One species, Volta's electric eel (Electrophorus voltai), can produce a discharge of up to 860 volts. In theory, if 10 such eels discharged at the same time, they could produce up to 8,600 volts of electricity—sufficient to power 100 light bulbs.
Mimicking Mother Nature
For soft robotics or wearable electronics applications, soft and stretchy devices with tissue-like electronic properties are required. However, “It’s difficult to design a material that is both highly stretchable and highly conductive since those two properties are normally at odds with one another,” said co-author Stephen O’Neill of the University of Cambridge. “Typically, conductivity decreases when a material is stretched.” So he and his colleagues decided to model their jelly battery design on the layered structure of the electric eel's electrocytes. Whereas conventional electronics employ rigid materials with electrons to carry the charges, this battery would use ions as charge carriers, like the electric eels.

Hydrogels—3D polymer networks composed of 60 percent water—were the obvious choice since they confer the ability to precisely control mechanical properties and can mimic human skin. They are usually made of neutrally charged polymers, but O'Neill et al. added a charge to their polymers, altering the salt component to make them sticky enough to squish together into multiple layers. This builds up a larger energy potential.
The stickiness of the hydrogels comes from the reversible bonds that form between the different layers, thanks to barrel-shaped molecules that act a bit like "molecular handcuffs," per the authors. So, the jelly batteries can stretch without separating the layers and without any loss of conductivity. Furthermore, “We can customize the mechanical properties of the hydrogels so they match human tissue,” said co-author Oren Scherman. “Since they contain no rigid components such as metal, a hydrogel implant would be much less likely to be rejected by the body or cause the build-up of scar tissue.” That makes them promising for future biomedical applications.
Another stretchy battery
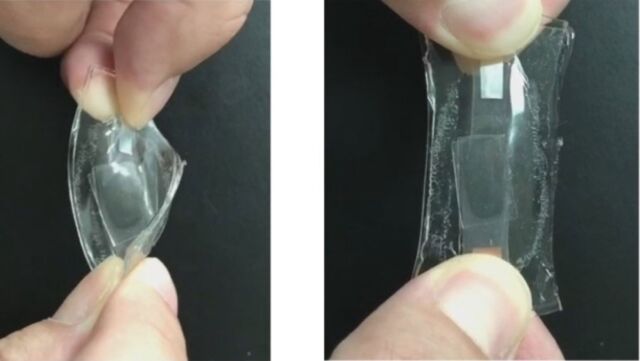
In related research, a new paper published in the journal ACS Energy Letters described the fabrication of a lithium-ion battery with stretchable components, including an electrolyte layer that can expand by 5,000 percent. The battery can retain its charge storage capacity after nearly 70 charge/discharge cycles. Rather than using a liquid electrolyte, a team of Chinese scientists incorporated the electrolyte into a polymer layer fused between two flexible electrode films.
The electrodes consisted of a thin film of conductive paste embedded with silver nanowires, carbon black, and lithium-based cathode or anode materials onto a plate. They applied a layer of flexible polydimethylsiloxane (used in contact lenses) on top of the paste, followed by a lithium salt, highly conductive liquid, and stretchy polymer ingredients. When zapped with light, all those components formed a solid rubber-like stretchy layer that could still transport lithium ions. This was topped with another electrode film, and the entire device was then sealed in a protective coating. This battery had a roughly six times higher average charge capacity at a fast-charging rate than a similar device with a traditional liquid electrolyte.
Science Advances, 2024. DOI: 10.1126/sciadv.adn5142 (About DOIs).
ACS Energy Letters, 2024. DOI: 10.1021/acsenergylett.4c01254 (About DOIs).
reader comments
20